Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
Amphoteric species are the species or compounds that act as acid as well as a base depending on the condition. Ammonia (nh 3) has an amphoteric nature as it acts as a weak base when reacts with acidic compounds and forms conjugate acid (nh 4+) by accepting the proton. Also, ammonia acts as a weak acid when reacts with a stronger base like oh. Why is ch3nh2 a weak base?
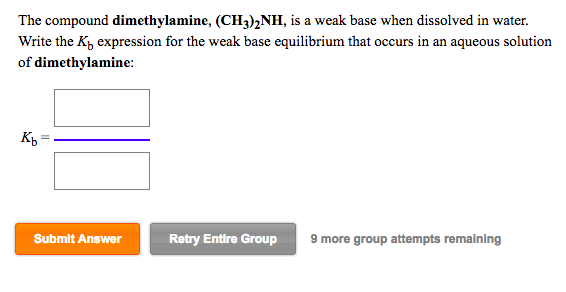
If you see a nitrogen in a compound and it has a lone pair, the compound is a weak base. Ch3nh2 is a weak base. By the definition of a bronsted base, it is a proton acceptor. Is ch3 2nh a strong or weak base? Dimethylamine ((ch3)2nh) is a weak base. A weak base is one that does not completely dissociate in water. It leaves only a small proportion of hydroxide ions and the concerned basic radical in the resulting aqueous solution. A large proportion of dissociated base molecules. Thus, the equilibrium for the reaction is (ch₃)2nh (aq) + h₂o (l) ⇄ (ch₃)2nh₃⁺ (aq) + oh⁻ (aq).
Solved: The Compound Dimethylamine, (CH3)2NH, Is A Weak Ba... | Chegg.com

Solved: The Compound Dimethylamine, (CH3)2NH, Is A Weak Ba... | Chegg.com

Use the References to access important values if | Chegg.com

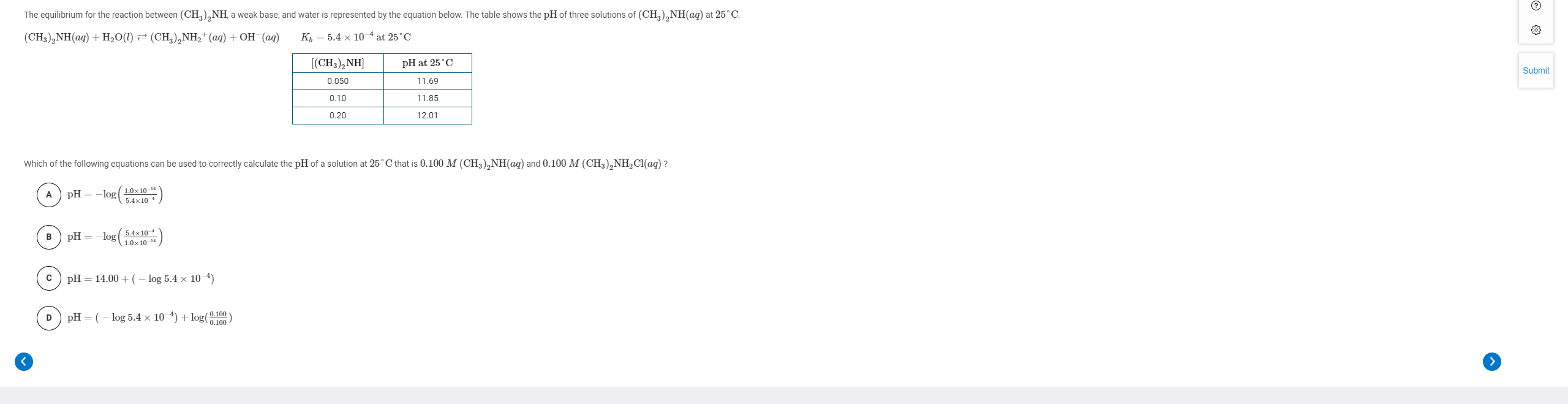
Solved: The Equilibrium For The Reaction Between (CH3)2NH(... | Chegg.com
Answered: The compound dimethylamine, (CH3),NH,… | bartleby
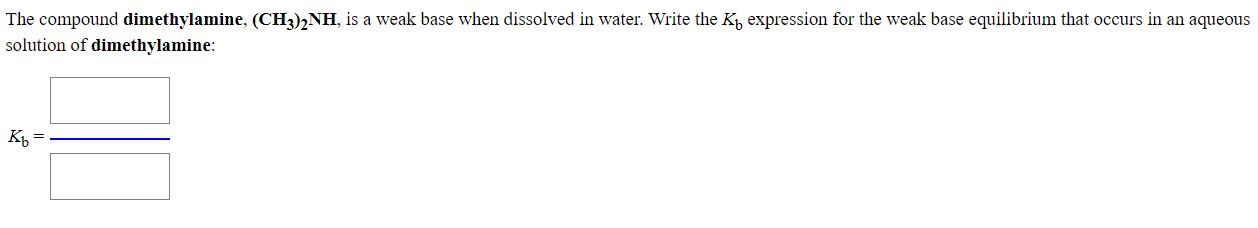
Answered: Classify each substance as a strong… | bartleby
Solved: The Equilibrium For The Reaction Between (CH3)2NH(... | Chegg.com

A solution is prepared at 25 °C that is initially 0.49 M in

The compound dimethylamine, (CH3)2NH, is a weak base | Chegg.com

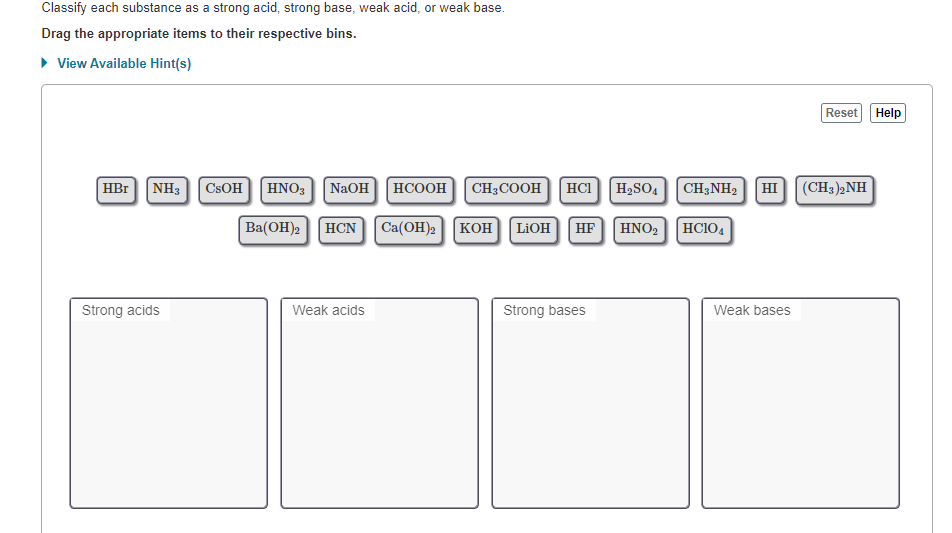
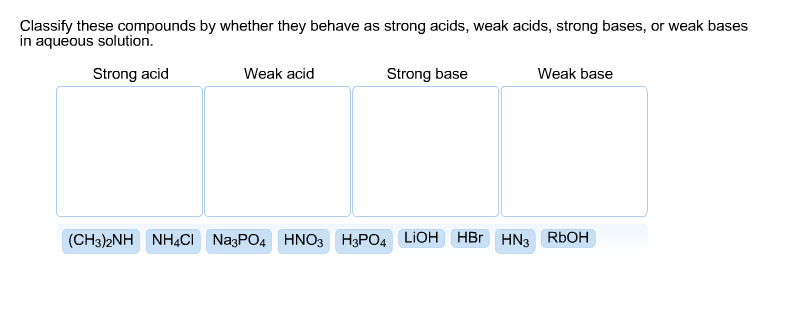
Solved: Classify These Compounds By Whether They Behave As... | Chegg.com

EmoticonEmoticon