Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
How do you calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level? 4) write the formula/expression for energy of electron in the n th orbit of hydrogen atom. 5) what is the kinetic energy of n th orbit of hydrogen atom. 6) how do you calculate the total energy of electron in the n th stationary orbit of hydrogen atom?
Calculate the energy of an electron in the n = 4 level of a hydrogen atom. Energy = j submit answer retry entire group 9 more group attempts remaining ted question : Calculate the energy of an electron in the n = 4 level of a hydrogen atom. Chemistry questions and answers. Calculate the energy of an electron in the n= 1 level of a hydrogen atom. Energy = joules calculate the energy for the transition of an electron from the n=2 level to the n=4 level of a hydrogen atom. Ae = joules is this an absorption (a) or an emission (e) process ? Calculate the energy for the transition of an electron from the n = 2 level to the n = 4 level of a hydrogen atom. E = ____joules is this an absorption (a) or an emission (e) process ?
Solved: Calculate The Energy Of An Electron In The N = 5 L... | Chegg.com
quantum mechanics - What is the largest wavelength that can excite an

Solved: Calculate The Energy Of An Electron In The N = 5 L... | Chegg.com
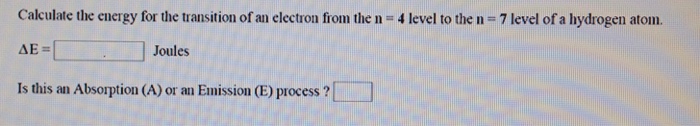
terminology - Is there a specific name for the highest energy state in

Solved: Calculate The Energy Change When An Electron Falls... | Chegg.com
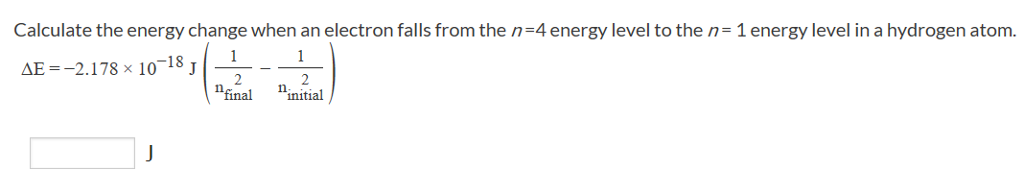
Photon Energy Level Equation - Tessshebaylo

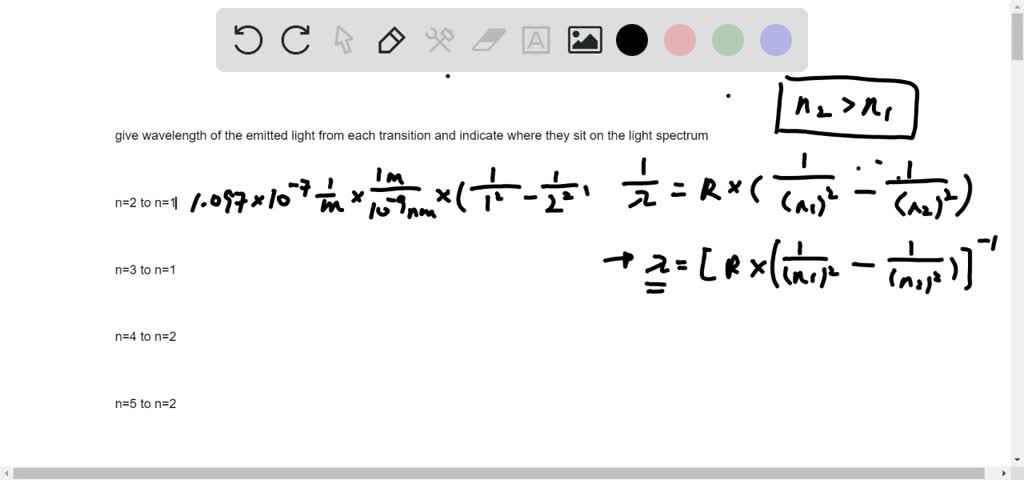
Solved: Calculate The Wavelengths Of Several Lines In The | Chegg.com
Solved: Calculate The Energy Of An Electron In The N-1 Lev... | Chegg.com

Calculate the wavelength of the light emitted whe…
Calculate the energy of an electron in the n = 4 | Chegg.com
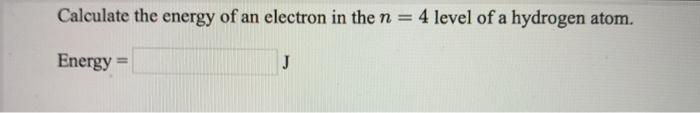
EmoticonEmoticon