
Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
Why does water have a higher boiling point than expected? The heat energy supplied to the matter is necessary to break the covalent bonds between the atoms. The water molecule is made up of hydrogen and oxygen atom. Since hydrogen has only one valence electron, it is a highly reactive element and forms a strong covalent bond and an enormous.
High boiling point and low melting point. Water has strong hydrogen bonds between molecules. These bonds require a lot of energy before they will break. Water has a high boiling point because its molecules are bound together by hydrogen bonding, which is a very strong intermolecular force. It takes more kinetic energy, or a higher temperature, to break the hydrogen bonding between water molecules, thus allowing them to escape as steam. Boiling of a liquid involves increasing the kinetic energy. Water has an unusually high boiling point for a liquid. Water is made up of oxygen and hydrogen and can form hydrogen bonds, which are particularly strong intermolecular forces. These strong intermolecular forces cause the water molecules to “stick” to one another and resist transition to the gaseous phase.
Why does water has a higher boiling point than HF | Hydrogen bonding in

Water in Space: What Happens? | ScienceBlogs
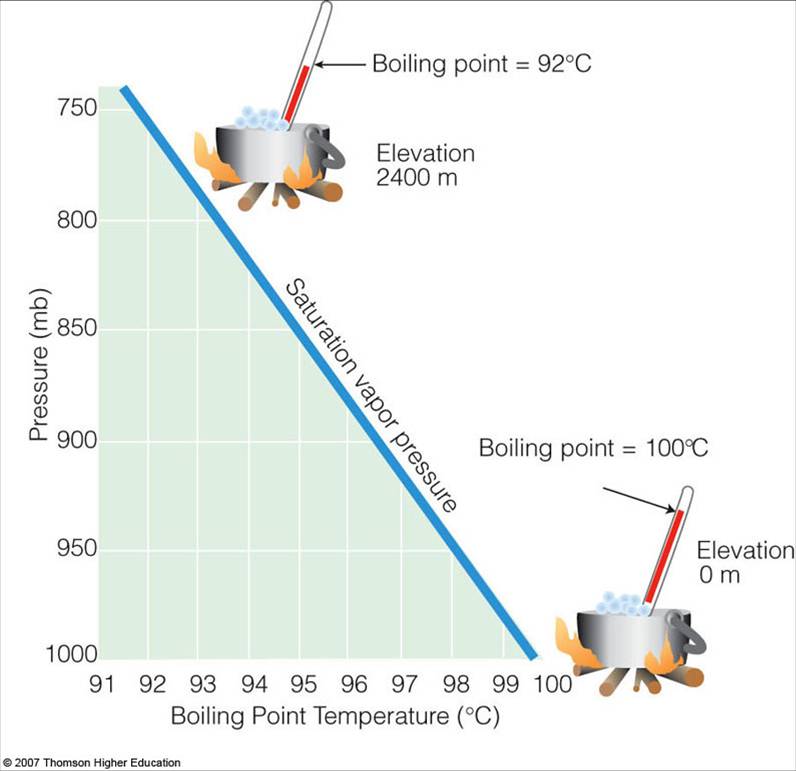
How do atmospheric pressure and elevation affect boiling point? | Socratic
Melting and Boiling Point - Careers Today

Perfect Interlude: Why Does Water Have A High Boiling Point

What Temperature Does Water Boil At? Boiling Point & Elevation

Perfect Interlude: Why Does Water Have A High Boiling Point

Perfect Interlude: Why Does Water Have A High Boiling Point

inorganic chemistry - Why does hydrogen fluoride have a boiling point

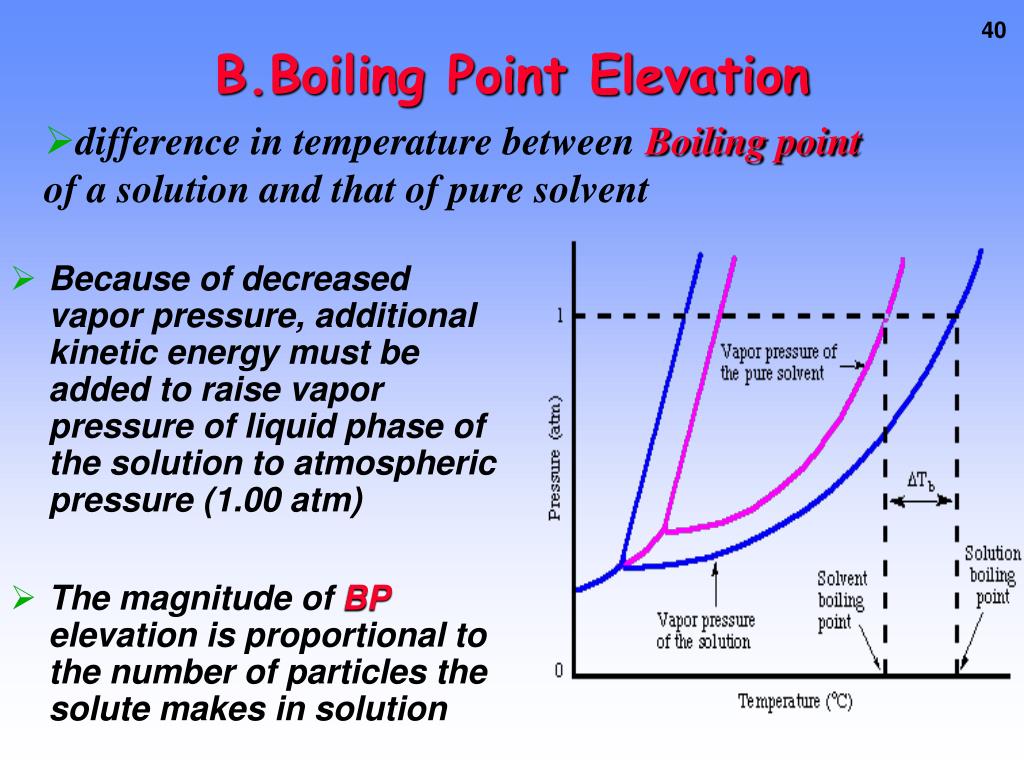
PPT - Physical Properties of Solutions Review and Colligative
EmoticonEmoticon