
Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
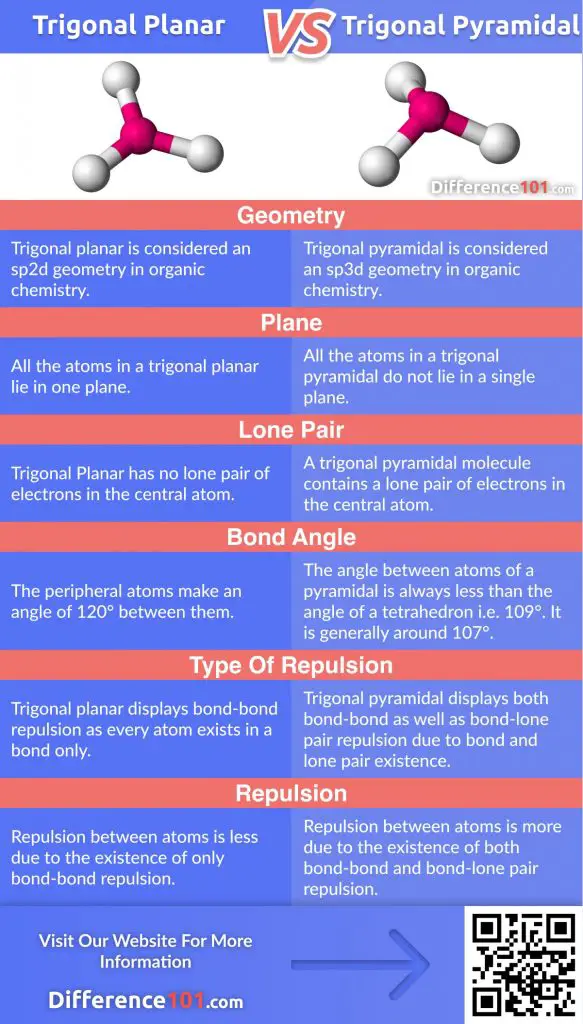
Molecules with the trigonal planar shape are triangular and in one plane or flat surface. Atoms in trigonal pyramidal are not in one plane. A molecule with an ideal trigonal planar geometry has an angle of 120 o between the peripheral atoms. The bond angle in a trigonal pyramidal is around 107 o.
Trigonal planar does not have a lone pair of electrons while trigonal pyramidal has a lone pair in the central atom. Also, all the atoms of a planar molecule lie in the same plane unlike the atoms of pyramidal. 1 what is trigonal planar shape?; 2 how do you know if a molecule is trigonal planar?; 3 why is it called trigonal planar?; 4 what’s the difference between trigonal pyramidal and trigonal planar?; 5 is trigonal planar 2d or 3d?; 6 what does trigonal pyramidal look like?; 7 is it trigonal or triangular?;
molecular structure - What's the difference between a tetrahedron and a

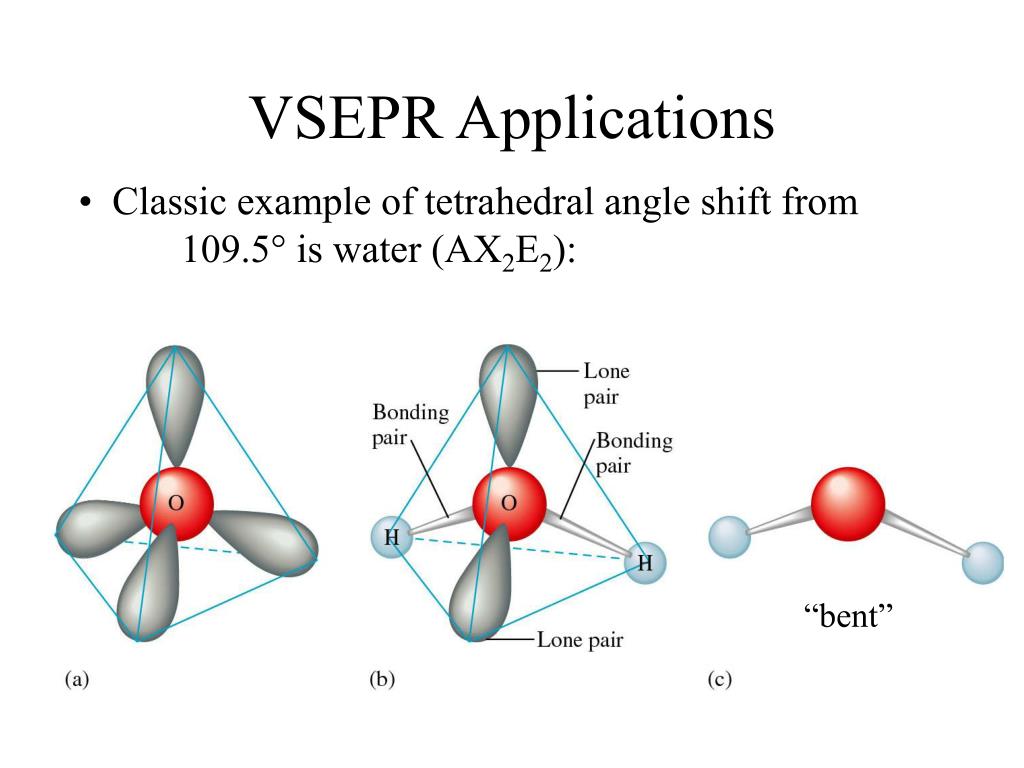
VSEPR PowerPoint - Tetrahedral, Trigonal Bipyramidal, etc.
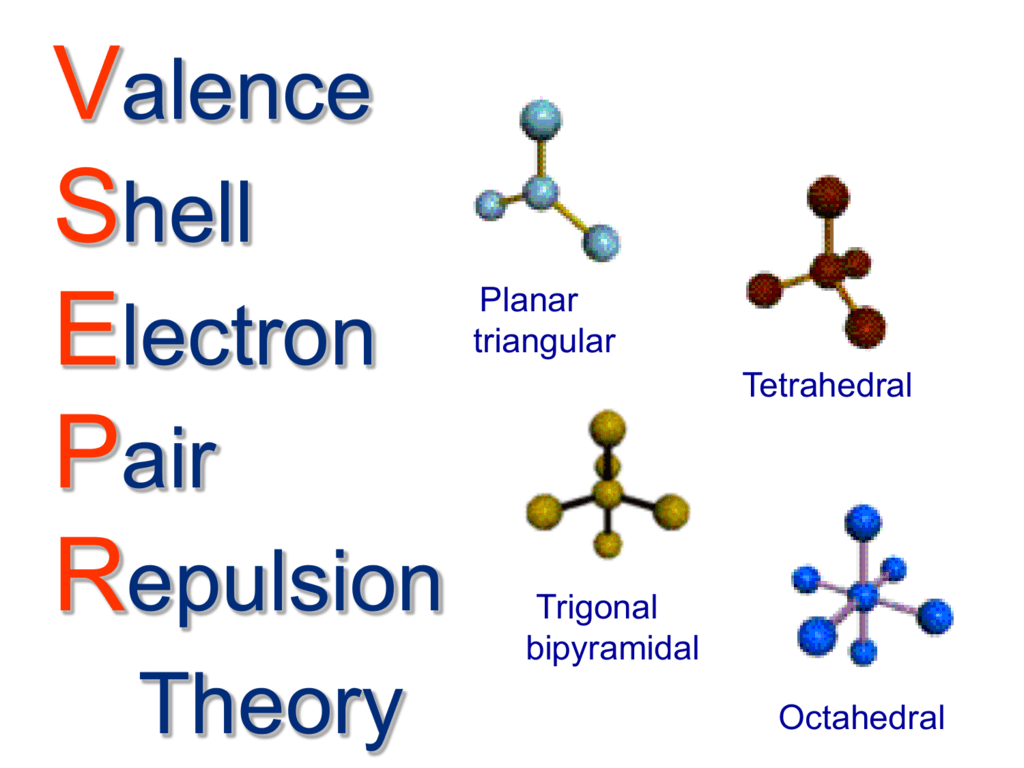
Josh's AP Chem Blog: 10/7/13-10/11/13

Trigonal Planar vs Trigonal Pyramidal: 6 Key Differences | Difference 101
8.8: Dipole Moments - Chemistry LibreTexts
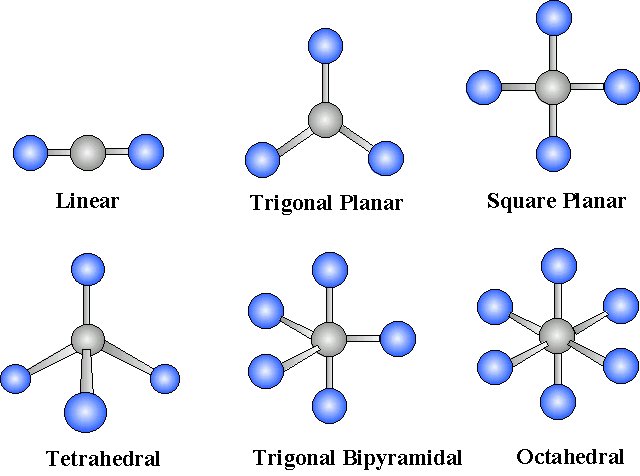
Molecular Geometry with Resonance - Organic Chemistry Video | Clutch Prep

What is the difference between Tetrahedral and Trigonal Pyramid

Ch. 9 Molecular Geometry
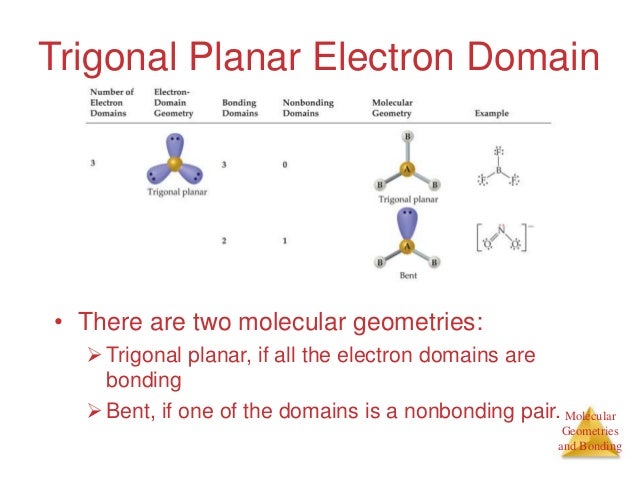
PPT - Lecture 25: VSEPR PowerPoint Presentation, free download - ID:515517
EmoticonEmoticon