Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
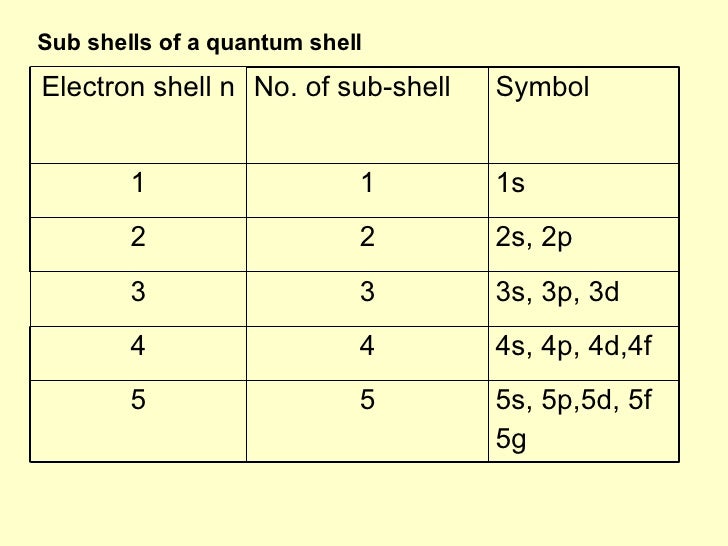
How many subshells are there in the shell with n 6? Posted on january 23, 2022 by ethika tasnim so, the n = 6 shell includes three subshells , namely 6s, 6p and 6d. 4s2 4p6 4d10 4f14. 5s2 5p6 5d10 5f14 5h18.
Each subshell can hold a different number of electrons. 14 the n number determines how many of the subshells make up the shell. For example, the 1st shell is made up of 1 subshell, s. It can therefore contain only 2 electrons. This gives seven extra orbitals, so for n = 4 there are 9 + 7 = 16 orbitals. Furthermore, how do you find the number of subshells? The shell and subshell are identical. Also to know, how many subshells are there in the shell with n 3? How many electrons can an n 6 shell theoretically hold?
SOLVED:How many subshells are in each shell: n=1,…
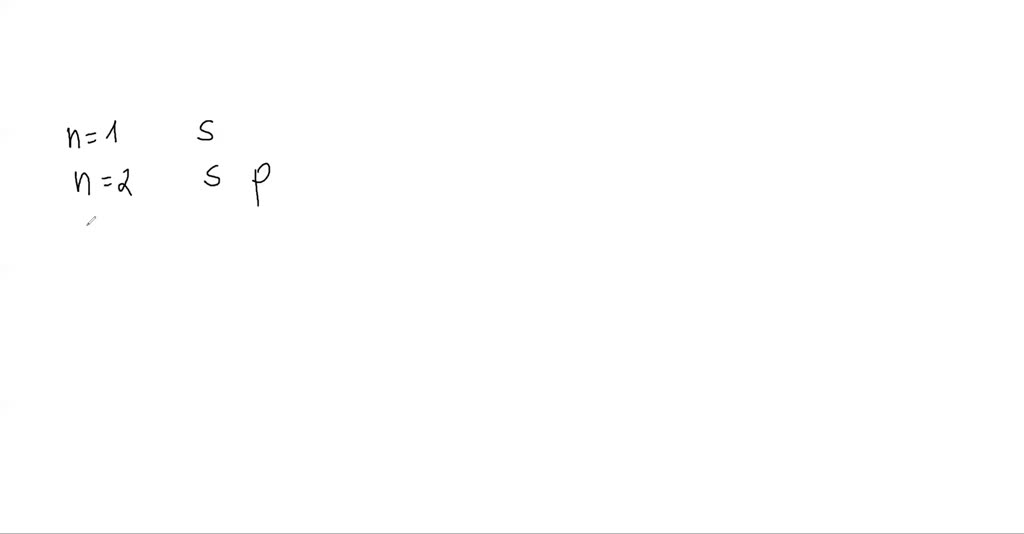
How many Subshells are there in the shell with n 3?

Chapter 7 notes

Elect
How many orbitals in an atom could have these sets of quantum numbers

CH150: Chapter 2 – Atoms and Periodic Table – Chemistry
Give all possible ml values for orbitals that have each of t

EmoticonEmoticon