
Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
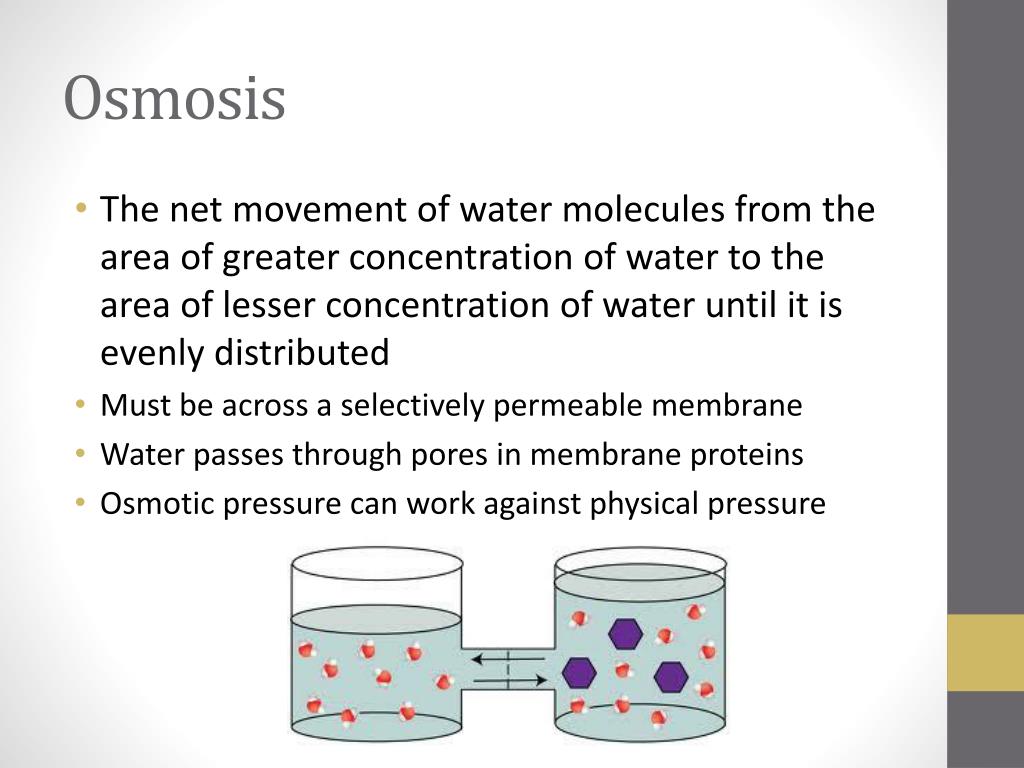
Which of the following would result in no change in osmotic pressure across a membrane? The solutes can diffuse through the pores and the concentration of solutes is the same on both sides of the membrane In 1951, the dutch physical chemist staverman provided a quantitative expression for the osmotic pressure across nonideal membranes, which was based on the following reasoning: Let us perform the ultrafiltration experiment illustrated in fig.
Which of the following would result in no change in osmotic pressure across a membrane? The solutes can diffuse through the pores and the concentration of solutes is the same on both sides of the membrane. With the experimental conditions set at 10 mm glucose and 9 mm albumin, and the 200 mwco membrane in place, which of the following is true? Osmotic pressure is the pressure required to stop water from diffusing through a membrane by osmosis. It is determined by the concentration of the solute. Water diffuses into the area of higher concentration from the area of lower concentration. When the concentration of the substances in the two areas in contact is different, the substances. The osmotic pressure can be approximated by using the following formula: To be applied to a solution to prevent the inward flow of water across a semipermeable membrane.
Osmosis & Osmotic Concentration - Lilach's Investigations

Figure 1:Peritoneal function in clinical practice: the importance of
Diffusion Osmosis for Moodle
PPT - Transport Across Cell Membranes PowerPoint Presentation - ID:2180239
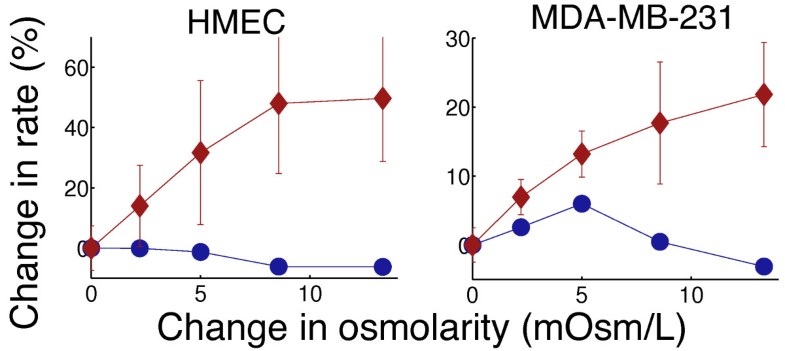
Separation of metabolic supply and demand: aerobic glycolysis as a
Solution & Colligative Properties | Question Bank for Class 12

Animal Cell Reaction To Hypertonic Solution / Hypotonic Solution

[Full text] Antimicrobial Peptide AMP-17 Affects Candida albicans by
![Which Of The Following Would Result In No Change In Osmotic Pressure Across A Membrane? [Full text] Antimicrobial Peptide AMP-17 Affects Candida albicans by](https://www.dovepress.com/cr_data/article_fulltext/s250000/250278/img/IDR_A_250278_O_F0006g.jpg)
How do oncotic and hydrostatic pressure differ? - Quora
Biology Archive | May 19, 2017 | Chegg.com

EmoticonEmoticon