
Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
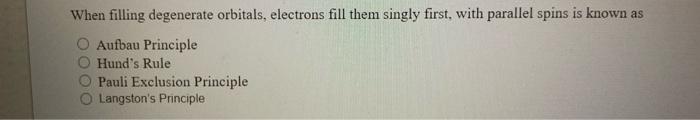
111) when filling degenerate orbitals, electrons fill them singly first, with parallel spins. What is this known as? A) pauli exclusion principle. D) heisenberg uncertainty principle.
8) when filling degenerate orbitals electrons fill them singly first; With parallel spins is known a) pauli exelusion principle b) hund's rule c) aulbau principle d) heisenberg uncertainty principle 9) a triple covalent bond contains a) 0 pairs b) pair c) 2 pairs d) 3 pairs e) 4 pairs of electrons_ Electrons fill them singly first. When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as a) pauli exclusion principle b) hund's rule It states that, when orbitals of equal energy (such as px, py, pz) are available electrons, they fill orbitals singly to keep the spin parallel. For example, n(z=7)= 1s2, 2s2, 2px2, 2py1 (wrong) n. 100% (13 ratings) correct option : B hund's rule hund's rule states that: Every orbital in a sublevel is singly occupied b.
Solved: Chapter 8 Assignment 1 Name: 1) No Two Electrons C... | Chegg.com

Orbital Diagram Of Ti2+

Solved: When Filling Degenerate Orbitals, Electrons Fill T... | Chegg.com
Chapter...D) [Kr]
![When Filling Degenerate Orbitals, Electrons Fill Them Singly First, With Parallel Spins Is Known As Chapter...D) [Kr]](https://s3.studylib.net/store/data/008758548_1-5cb6ca63160320ee288f5c0c2564d9db-768x994.png)
Solved: Chapter 8 Assignment 1 Name: 1) No Two Electrons C... | Chegg.com

Homework Kotz Chapter 7 (1) - Homework Kotz Chapter 7 Name 1 No two

Solved: 3. Which Period 3 Element Has The Following Ioniza... | Chegg.com

Chapter...D) [Kr]
![When Filling Degenerate Orbitals, Electrons Fill Them Singly First, With Parallel Spins Is Known As Chapter...D) [Kr]](https://s3.studylib.net/store/data/008758548_1-5cb6ca63160320ee288f5c0c2564d9db.png)
Solved: Chapter 8 Assignment 1 Name: 1) No Two Electrons C... | Chegg.com

Orbital Diagram Of Ti2+

EmoticonEmoticon