Axolotl My Time Has Come To Burn I Invoke The Ancient Power That I May Return, A-X-O-L-O-T-L my time has come to burn i invoke the ancient power that i may return, 1.08 MB, 00:47, 441,539, Jesus the Gamer1000, 2021-04-10T00:01:36.000000Z, 19, MY TIME HAS COME TO BURN by 20percentcooldash on DeviantArt, 20percentcooldash.deviantart.com, 862 x 927, png, burn come deviantart, 20, axolotl-my-time-has-come-to-burn-i-invoke-the-ancient-power-that-i-may-return, KAMPION
In other words, two electrons (in the same atom) cannot have identical quantum numbers. For each m l there are only two allowed values of m s, namely m s = + 1 2 and m s = − 1 2. Thus, each set of { n, l, m l } can only describe two electrons. In your case, we have two electrons with n = 5, l = 3, m l = − 1:
In an atom both 4s (n = 4 l = 0) and (n = 3 l = 1) orbitals can have n + l =4. Since four orbitals are involved they can have maximum of eight electrons. How many electrons can fit in the orbital for which n? Each orbital can occupy a maximum of 2 electrons 8 electrons as you know, each electron has a unique set of quantum numbers that describes its exact location in an atom. In your case, you are given two qunatum numbers, n, the principal quantum number, and m_l, the *magnetic quantum number, and are sked to determine how many electrons can share these two quantum numbers in an atom. N=5 tells you that your quantum number is 5. L=3, tells you that it is 5f. Therefore, it can only hold 2 electrons.
[Solved] How many electrons can be described by the quantum numbers n
Solved: How Many Electrons In An Atom Could Have These Set... | Chegg.com
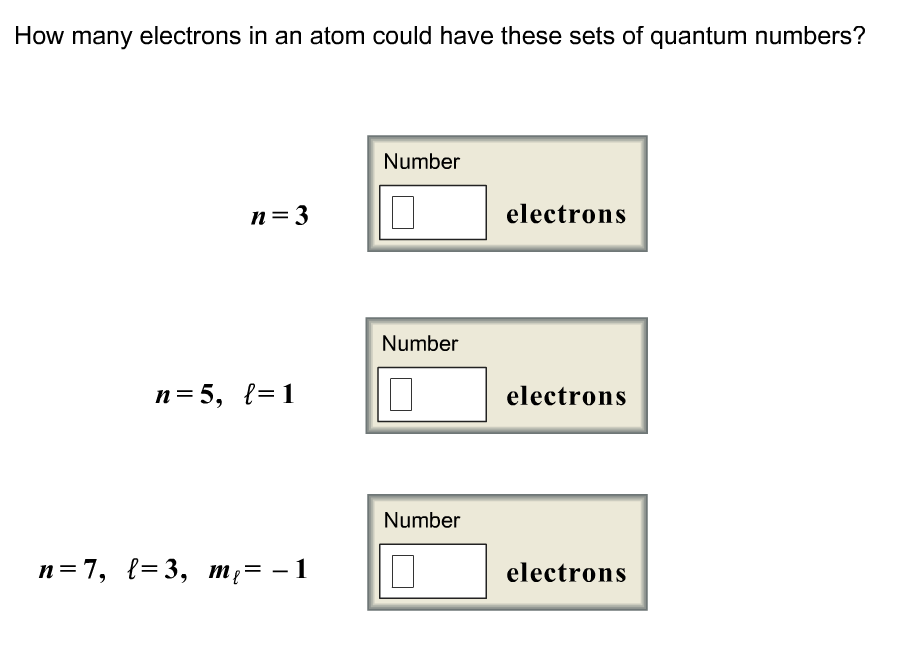
Orbitals electron-configuration
[Solved] 9 1 point How many known elements have atoms, in their ground
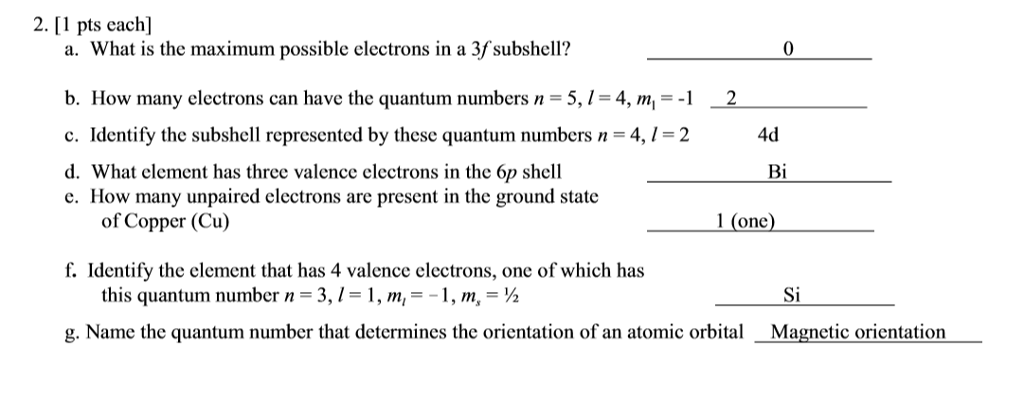
Solved: What Is The Maximum Possible Electrons In A 3f Sub... | Chegg.com
Solved: How Many Electrons In An Atom Could Have These Set... | Chegg.com

How many electrons in an atom could have these sets | Chegg.com
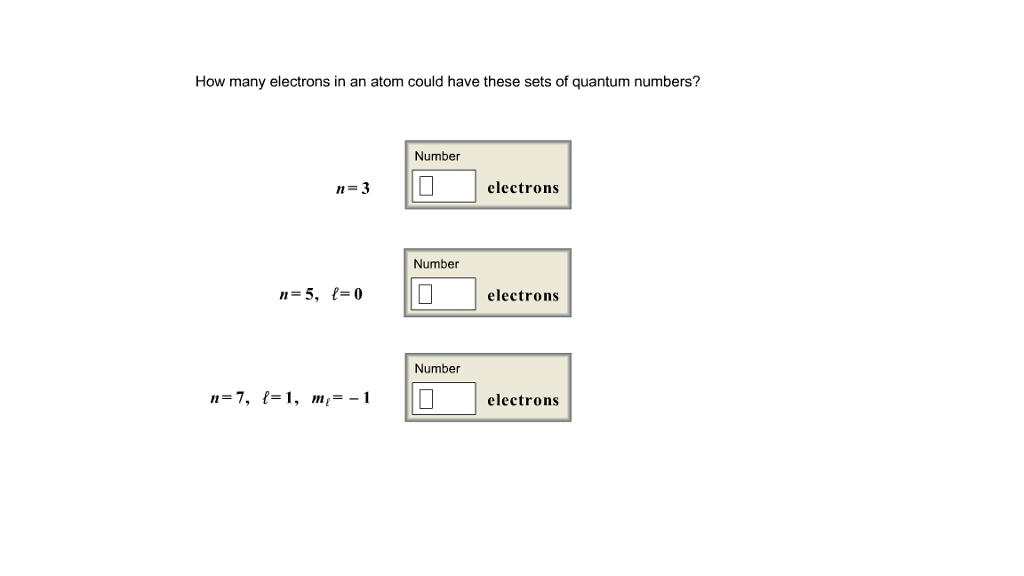
How many electrons could occupy a subshell with the | Chegg.com

Quantum Numbers - Chemistry Video | Clutch Prep

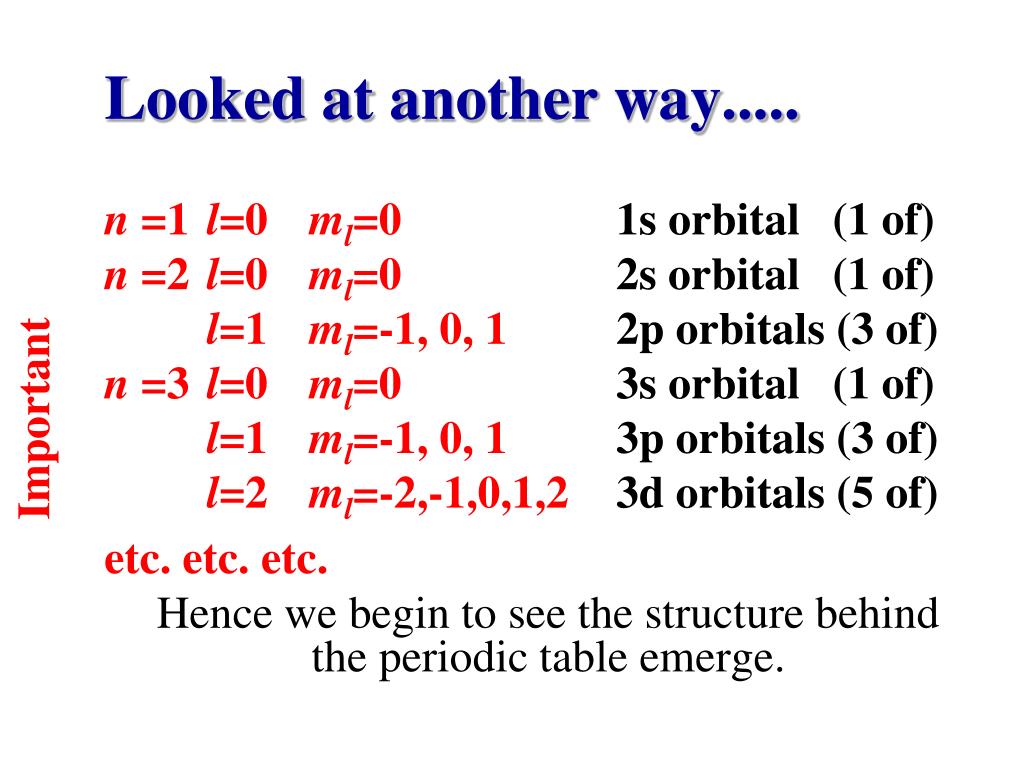
PPT - Atomic Structure and Periodic Trends PowerPoint Presentation
EmoticonEmoticon